[Study News] HKU secured three spots in the Top 10 Innovation & Technology News in Hong Kong 2025
05/01/2026

[Study News] HKU secured three spots in the Top 10 Innovation & Technology News in Hong Kong 2025
05/01/2026

.jpg)
The University of Hong Kong secured three spots in the "Top 10 Innovation & Technology News in Hong Kong 2025”, organized by the Beijing-Hong Kong Academic Exchange Centre. The winners were selected through an online public vote involving over 4,000 participants from academia, research, education, and other sectors.
One of the awarded projects is the innovative “dual immunotherapy” for advanced liver cancer, led by Professor Thomas Yau, Clinical Associate Professor in the Centre of Cancer Medicine and Department of Medicine, School of Clinical Medicine, HKUMed. The novel approach combines two drugs, nivolumab and ipilimumab, to activate the immune system against advanced liver cancer cells. Professor Yau led his team, in collaboration with the HKU Clinical Trials Centre, to launch clinical trials starting from 2016 to evaluate its efficacy, safety and the effectiveness in prolonging patient survival. It has confirmed that this new therapy significantly improves survival rates and tumour control. This therapy has successfully received approvals from the US FDA, EMA, and China’s NMPA for use as a first-line treatment for adult patients with unresectable or metastatic liver cancer.
For more details on this research, please visit the website below: https://www.hku.hk/press/press-releases/detail/28418.html
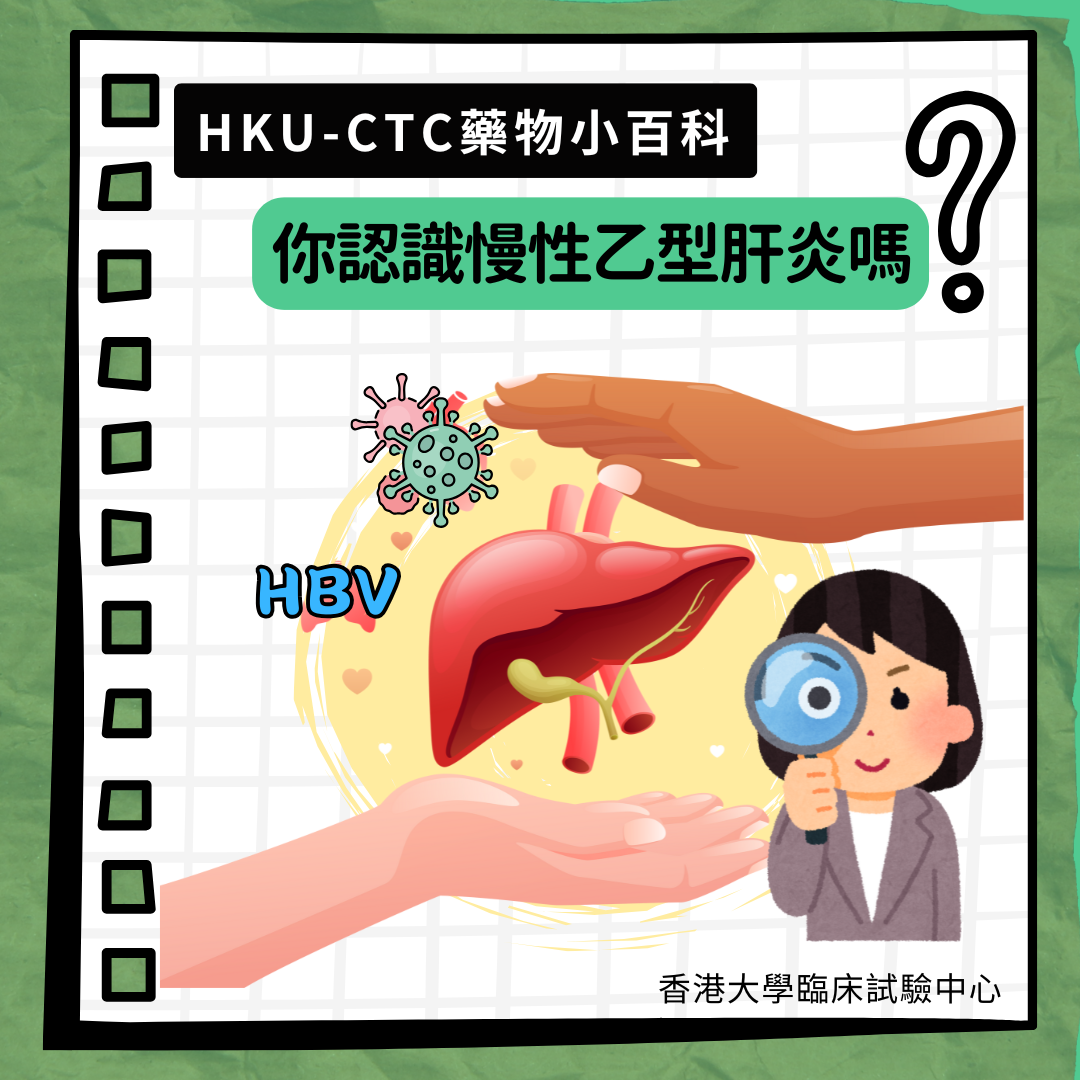
[New Drug Journey] Towards Functional Cure for Hepatitis B: Explore Potentials of Gene Therapy
23/12/2025

[New Drug Journey] Towards Functional Cure for Hepatitis B: Explore Potentials of Gene Therapy
23/12/2025
In Hong Kong, approximately 410,000 people are suffering from chronic hepatitis B (CHB). However, the infection of hepatitis B virus (HBV) may remain asymptomatic at early stage. If appropriate treatment is not received promptly, the virus may replicate within the liver, gradually damage liver cells and develop secondary to cirrhosis, liver cancer or serious liver damage. The current available treatment options for HBV patients is adopting oral medication to suppress the virus development. Unfortunately, these drugs mostly serve to control the disease long-term rather than eliminate the virus completely, meaning patients often require lifelong medication to prevent disease progression and reduce the risk of virus transmission.
A research team from the Department of Medicine, School of Clinical Medicine, LKS Faculty of Medicine, the University of Hong Kong (HKUMed) has been actively exploring innovative treatment strategies by targetting the root cause of virus infection. Through gene therapy, they aim to eradicate HBV from human's body and work towards the goal of achieving "functional cure" for patients. This approach may not only help patients break free from the burden of lifelong medication but also reduce the risks of developing liver cirrhosis and liver cancer later on.
To learn more about the latest research directions in CHB treatment, watch the team's interview video here: https://www.youtube.com/watch?v=bqXKC3NTloM
[Trial Results Release] New ‘Dual Immunotherapy’ in Liver-cancer Patients Receives International Approvals
18/08/2025

[Trial Results Release] New ‘Dual Immunotherapy’ in Liver-cancer Patients Receives International Approvals
18/08/2025

Thank you to all the patients who participated in the trial targetting the use of a ‘dual immunotherapy’ combination—nivolumab and ipilimumab (NIVO+IPI) — in the treatment of liver cancer patients between 2020 to 2021. This innovative treatment has proved to significantly improve survival rates and tumour control compared to current first-line treatments, lenvatinib and sorafenib. Recently it was approved by the US Food and Drug Administration (FDA), the European Medicines Agency (EMA) and China's National Medical Products Administration (NMPA) for global use, offering new hope to liver cancer patients worldwide.
[Centre Activity] HKUMed x QMH Summer Attachment Programme 2025
14/08/2025
We were delighted to welcome a group of secondary school students from the HKUMed x QMH Summer Attachment Programme at the HKU Phase 1 Clinical Trials Unit on August 7, 2025.
The visit began with an insightful introduction to clinical trials and HKU-CTC, followed by a guided tour of our Phase 1 Unit. The students engaged in discussions and got a glimpse of the day-to-day processes behind clinical research. It was gratifying to see their curiosity and active engagement as they explored the possibilities of a future in medicine.
We are looking forward to nurturing more innovators of tomorrow!
[Centre Activity] Happy International Clinical Trials Day!
20/05/2025


In celebration of the International Clinical Trials Day, a global celebration honoring a Scottish physician James Lind, who launched the first documented clinical trial on scurvy on May 20, 1747, we set up a booth at Queen Mary Hospital this year to showcase the story behind this special occasion, and to raise social awareness on the significance of clinical trials in medical advancement!
Other than the informative posters educating the public on the close association between new drug discovery and clinical trials, we had also prepared thoughtfully-designed souvenirs to interest participants who passed by our booth! One of our Panda Ambassadors, Dr Hong also joined us at the booth to bring more smiles and energy to the event, creating a welcome space for dialogues with all the participants. We hope you have all enjoyed, and look forward to bringing more medical knowledge to you all soon!
[Trial Results Release] Inavolisib-Based Therapy in PIK3CA-Mutated Advanced Breast Cancer Approved by U.S. FDA
24/01/2025

[Trial Results Release] Inavolisib-Based Therapy in PIK3CA-Mutated Advanced Breast Cancer Approved by U.S. FDA
24/01/2025
Thank you to all the patients who participated in the oral medication study related to Inavolisib-based therapy in PIK3CA-mutated advanced breast cancer (CTC1946) between 2020 and 2023. We have the honor to inform you that the clinical data of the Phase 3 clinical trial has been submitted to the U.S. Food and Drug Administration (FDA) and the oral medication has been approved, paving ways for new treatment targeting breast cancer.
[Centre Activity] Clinical Research Governance World Conference 2025
17/01/2025
.jpg)
Clinical Research Governance World Conference 2025 was successfully concluded on January 16, 2025. With over 200 on-site and 800 online registrations, the engagement from all these indsutrial experts was what make the event special. It is our honour to have Professor Lo Chung-mau, Secretary for Health of HKSAR, and Professor Max Shen, Vice-President (Research) of The University of Hong Kong, deliver the opening remarks. Their insights set the tone for a productive conference.
We hope to see you all in the our future events again!
[Centre Activity] Visit from Secondary School Students at HKU Phase 1 Centre
03/12/2024
We are delighted to welcome a group of secondary school students visiting our HKU Phase 1 Clinical Trials Centre on November 22, 2024. It was an excellent opportunity to enlighten the next generation about the vital role of clinical trials in progressing public health.
The information session also included introduction to the diversified research projects that our Centre has been working on, sparking their interests in the clinical trials industry and research outputs that can benefit the community.







.jpg)

