[Pharma Tips] I missed a dose, what should I do?
08/10/2022

In the modern days, people usually live a busy life. It is not uncommon for them to miss a dose of medication. What should they do? Take the missed dose immediately, or double up the amount at the next dose?
Generally speaking, for oral drugs, if it is only 2 hours since your missed dose, you can still make up for it. If it is over 2 hours, you should decide depending on the dosing frequencies.
- If the medicine is only taken once or twice daily and there is a few hours until the next dose, go ahead and take the missed dose then take the later doses as usual.
- If the medicine is taken three times or more a day, it is recommended to take your next dose at the normal schedule.
Bear in mind that never doubling up on the medication even you miss a dose. It might cause a sudden surge in drug concentration and increase the chance of getting unwanted adverse effects.
This is a general recommendation. For the differences in drug effects and health conditions of individuals, consult your doctor or pharmacist when questions arise.
COVID-19 Vaccine (Omicron Variant) Clinical Trial Exceeds Recruitment Target of 1800 Volunteers
28/09/2022

COVID-19 Vaccine (Omicron Variant) Clinical Trial Exceeds Recruitment Target of 1800 Volunteers
28/09/2022
After four months of dedicated effort, we have successfully completed the recruitment of volunteers. Over 1800 individuals participated in this clinical study and received the investigational vaccine, surpassing the targeted number of participants. We will continue to gather data from the vaccine study and plan to conduct the final analysis by the end of 2022, with the aim of expediting the introduction of the Omicron COVID-19 vaccine.
We extend our heartfelt gratitude to all those who actively took part in this research as participants and to those who shared research information with their family and friends. Your tremendous support is highly appreciated, as it demonstrates your willingness to accompany us on this journey of vaccine development.
[Pharma Tips] Why must some medicines be taken on empty stomach?
08/09/2022

Some think all medicines must be taken after meals. The correct way is to follow the directions on the medicine label. Why must some medicines be taken on empty stomach?
Empty stomach is defined as one hour before eating or 2 hours after eating. Some compositions in food might interact with the drug, affecting the absorption and lowering the effectiveness. Some might increase the amount of medicine in your blood to potentially dangerous levels.
A special example to share is the intake of bisphosphonates. Bisphosphonates is used to treat osteoporosis. It is recommended to take on am empty stomach in the early morning. After administration, the patient should not have food, drink or mediciations for at least 30 to 60 minutes, and stay upright for at least 30 minutes to prevent the drug from causing oesophageal irritation if stayed for too long.
[Pharma Tips] Why must some medicines be taken with or after food?
08/08/2022

We talked about taking medication on empty stomach last time. On the other hand, why must some medicines be taken with or after food?
There are several major reasons:
- To reduce the side effects of nausea or vomiting. Some food can relieve the nauseousness caused by the drug.
- To reduce the side effects of an upset stomach. If the drug causes side effects like indigestion, stomach inflammation or ulcers, having biscuits or a glass of milk before administration can relieve an upset stomach.
- To ensure best effect. Antacids are used to treat heartburn and indigestion, which usually occurs when food enters the stomach and produces gastric acids. It is most effective to take these medicines after eating.
- To ensure the medicine is not washed away. Mouthwashes and oral gels for treating mouth ulcers must be used after meals because they are washed away quickly when eating.
- To facilitate absorption. Some drugs need to be taken with food to lengthen its time staying in the stomach for better absorption.
- To help process the meal. Oral drugs for diabetes should be taken with food to reduce the blood glucose level and avoid low blood sugar after administration.
[Pharma Tips] Are antibiotics universal?
08/07/2022

Many people think antiobiotic is a panacea. When they have a fever or feel dizzy, they might take the leftover antibiotics to reduce waste and kill the bacteria inside the body. Once recovered, they will stop the medication. People usually mix up antibiotics with anti-inflammatory drugs which in fact, antibiotics should be used prudently.
Antibiotics are effective in treating bacterial infections and ineffective in treating other infections not caused by bacteria. They do not work for viral infections such as influenza. Do not take antibiotics without doctor’s prescription and always follow their instructions when taking antibiotics.
Doctors always tell the patients to finish a course of antibiotics treatment and do not stop the medication on their own. This is because the bacteria might not be completely killed. They might adapt to the antibiotics and create resistance. Further reproduction may lead to relapse. The same antibiotics may not work on the same bacteria again which lead to fewer treatment options. Appropriate use of antibiotics is very important or it may cause devastating consequences on our next generation.
[Study News] HKU medical research team starts large-scale clinical trial on COVID-19 Vaccine (Omicron Variant)
09/06/2022

[Study News] HKU medical research team starts large-scale clinical trial on COVID-19 Vaccine (Omicron Variant)
09/06/2022

A medical research team from the University of Hong Kong (HKU) started a large-scale clinical trial on a new COVID-19 inactivated vaccine (Omicron variant). The study is led by Professor Ivan Hung, Chief of Division of Infectious Diseases and Clinical Professor of Department of Medicine, LKS Faculty of Medicine, the University of Hong Kong (HKUMed) and HKU Clinical Trials Centre (HKU-CTC) is responsible for coordination and technical support.
A press conference was held on 31st May, 2022 to share with the public more about the details of the clinical trial. Professor Ivan Hung, Dr. Kelvin To (Chairperson and Clinical Associate Professor of Department of Microbiology, HKUMed) and Mr. Henry Yau (Managing Director of HKU-CTC) were invited. For more details about the press conference, please visit: https://hku.hk/press/news_detail_24616.html
As thousands of volunteers are needed for this study, if you are interested to join, please register via the link below:
https://redcap.link/joinctc2298
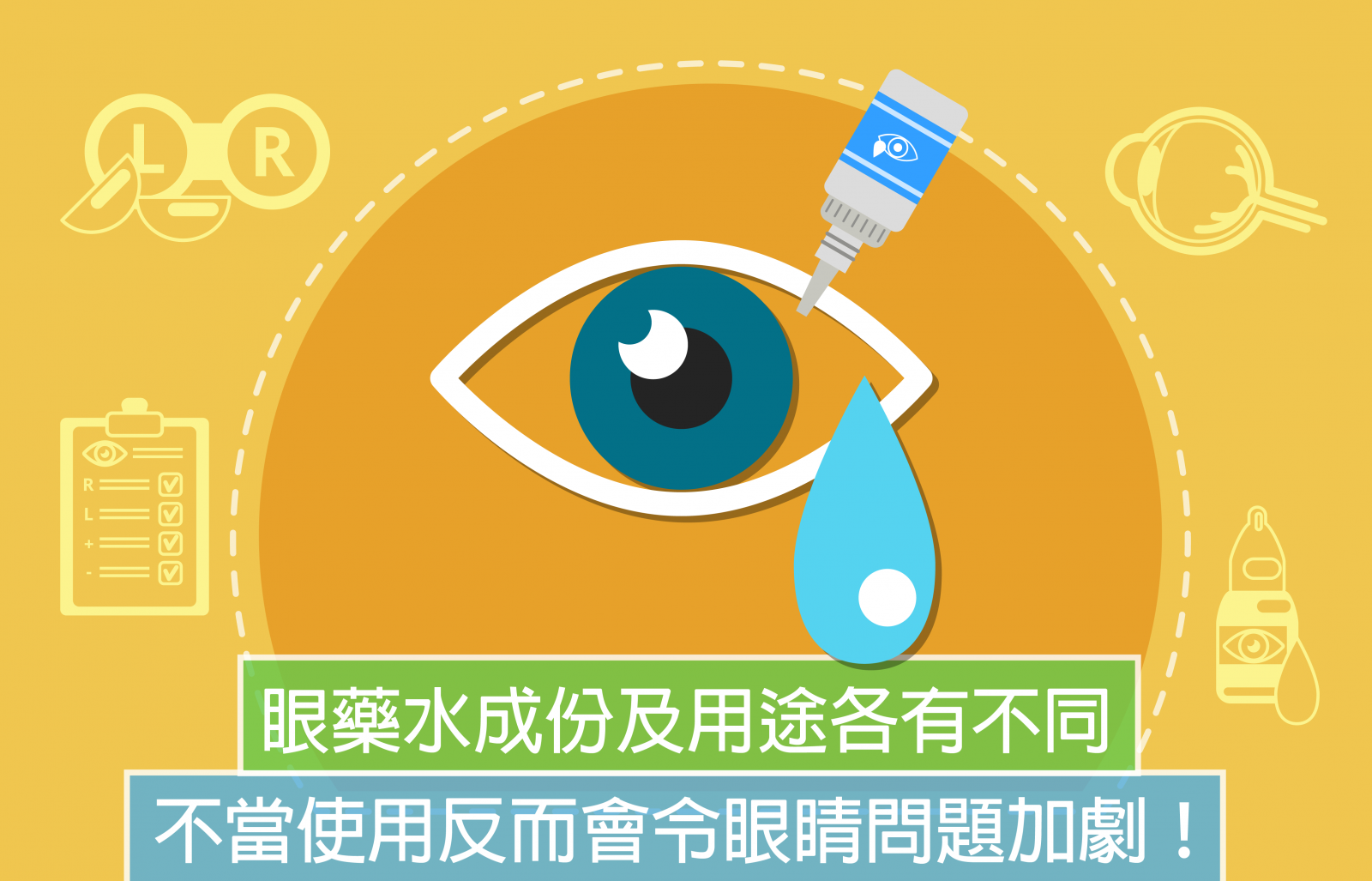
How to use eye drops properly?
08/06/2022
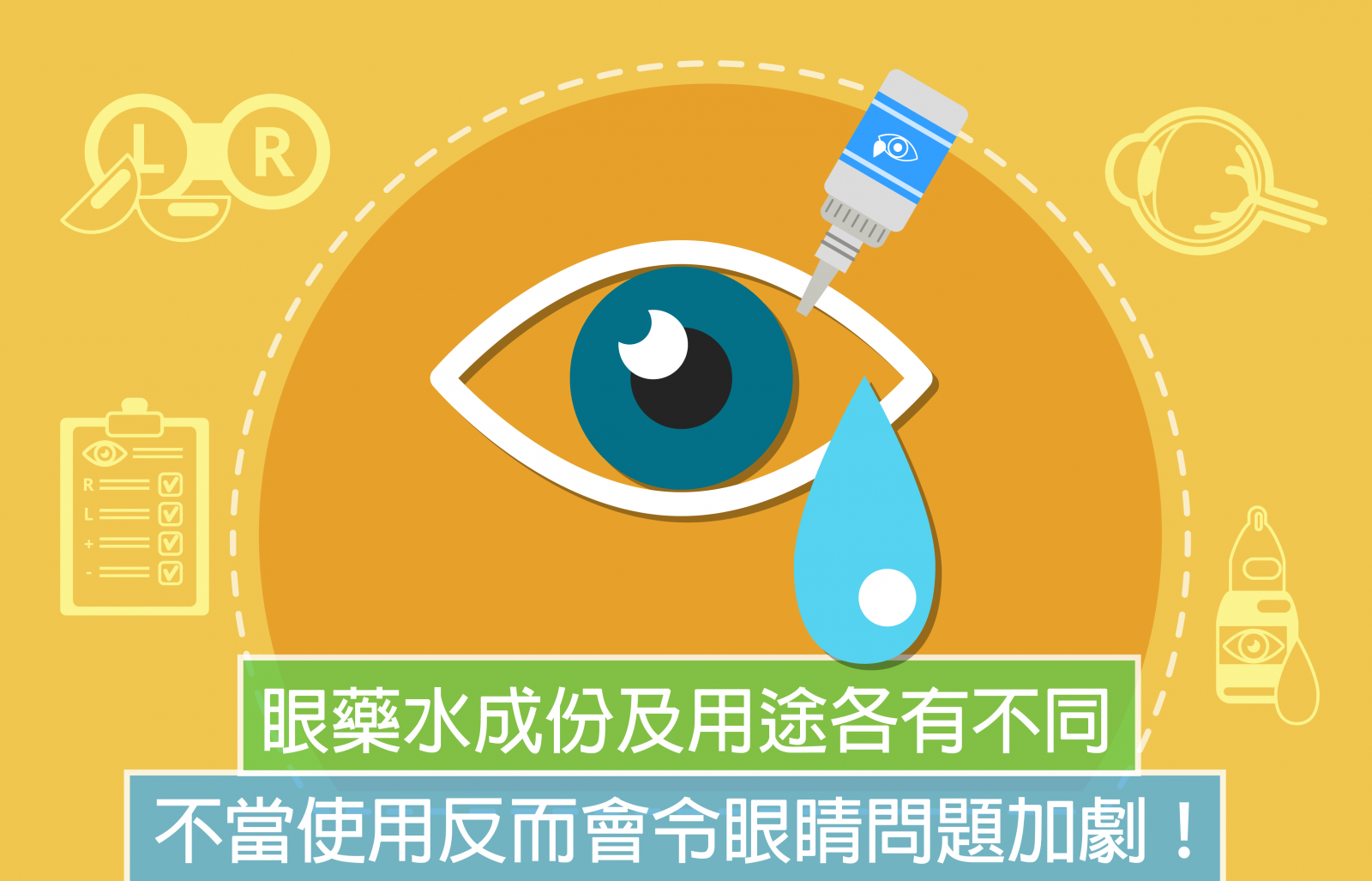
A lot of people traveling to Japan will purchase decently packaged eye drops. There are articles of “10 Must-Buy Japanese Eye Drops” on the internet, introducing how refreshing this is and how lubricating another is. However, have you realized that eye drops should be properly administered? Misuse of eye drops may fail to alleviate the discomfort, but instead worsen the symptoms!
There are many types of eye drops, such as antibiotic drops, pressure-lowering drops, steroid drops and artificial tear drops. They have different ingredients and serve various purposes.
- Antibiotic drops are used to treat bacterial infections. The patient should finish a whole course according to doctor’s directions. Misuse may lead to antibiotic resistance.
- Pressure-lowering drops are used for treating glaucoma. It can cause side effects so always follow doctor’s instructions when using it.
- Steroid drops are used to treat inflammation. Inappropriate or long-term use may elevate the intraocular pressure and develop glaucoma.
- Artificial tear drops are used to lubricate dry eyes and relieve discomfort. They contain no medicated ingredients. However, some drops are not preservative-free and may induce allergic reactions.
There was once an undiagnosed glaucoma patient who did not seek medical consultation but purchase and apply steroid eye drops on his own. It exacerbated the glaucoma. Therefore, if your eyes feel unwell, visit a doctor before any irreversible consequences happen.
[Pharma Tips] Can I take medications with beverages other than water?
08/05/2022

As an old saying goes, “Good medicine for health tastes bitter. But the truth is, the bitterness of a medicine makes it tough to swallow. When my younger self was taking pills, wild thoughts often pop up – can we take the medicines with beverages other than water, say, milk or juices?
Most pills or capsules should be taken together with water, as some drugs might interact with food or beverages. For example, some medicines should not be taken with milk. Calcium in dairy products interfere with certain medications and affects the absorption.
Other common example is grapefruit-drug interaction. No matter it is whole fruit, freshly squeezed juice or concentrate, the interaction comes in all forms and they can all inhibit an enzyme cytochrome P450 3A4 in liver and impacts the drug metabolism, hence leading to a high dose of drug in blood (eg. Statins, a cholesterol lowering drug). Some other citrus fruits like Seville oranges and bergamots will also cause the same interaction.
Phase 1 Study Shows Synergistic Anti-tumour Efficacy of Combination of New Drug and Chemotherapy for HCC, Warranting Further Phase II/III Studies
11/04/2022
Phase 1 Study Shows Synergistic Anti-tumour Efficacy of Combination of New Drug and Chemotherapy for HCC, Warranting Further Phase II/III Studies
11/04/2022
[Pharma Tips] How to determine the dosing interval?
06/04/2022

Do you follow the standard intervals indicated on the drug label and take your medicines on time? Why are some drugs only taken once daily, while some need to be taken for more than 4 times a day? Some people might also think that the more medicines one takes, the faster the recovery. Is it true?
Number of doses per day is affected by a bunch of factors, one of which is the metabolic rate of a drug in human body. If the metabolic rate is fast, the daily dose would be higher so as to maintain a steady drug concentration in your body. You should follow the instuctions and take the medications on time. Never take more or less medicines than prescribed. Overdose will cause toxicity, while insufficient dosage will affect the treatment.
The dosing interval is determined by the number of daily doses. Unless specified by your doctor, the dosing interval is as follows:
- 1 time a day: Every 24 hours and taken at the same time every day
- 2 times a day: Every 10-12 hours
- 3 times a day: Every 6-8 hours
- 4 times a day: Every 4-6 hours