[Study Completion] Successful Completion of Clinical Study for Sancuso® skin patch
20/12/2021

[Study Completion] Successful Completion of Clinical Study for Sancuso® skin patch
20/12/2021
[Centre News] International Clinical Trial Center Network (ICN) Steering Board Meeting and Annual General Meeting 2021
18/11/2021
[Centre News] International Clinical Trial Center Network (ICN) Steering Board Meeting and Annual General Meeting 2021
18/11/2021
The ICN Annual Steering Board Meeting 2021 took place on November 16, 2021 in the form of virtual meeting and was hosted by Cambridge Clinical Trials Unit (CCTU), Cambridge University Hospitals NHS Foundation Trust, one of the steering board members. During the meeting, all steering board members made decisions on key governance items. Mr. Henry Yau, ICN Chairperson cum Managing Director of HKU-CTC and Ms. Dr med Christiane Blankenstein, ICN Vice-Chairperson cum Director of Muenchner Studienzentrum, led the discussion on the strategic plan of ICN for the incoming year, which set out a direction for the Operations Team to work on in the year to come.
The ICN Annual General Meeting 2021 was held on November 17, 2021. Three experience sharing sessions were arranged and representatives from Cambridge University Hospitals and AstraZeneca were invited to talk about patient and public involvement as well as the influences of COVID-19 on clinical trials, which are both hot topics in 2021. Enthusiastic responses were received during the Q&A session. The next ICN Steering Board Meeting and Symposium will be hosted by Muenchner Studienzentrum, Technical University Munich, School of Medicine, in October 2022.
[Centre News] “A tour to HKU Phase 1 Clinical Trials Centre” Interview by Our Hong Kong Foundation
05/11/2021

[Centre News] “A tour to HKU Phase 1 Clinical Trials Centre” Interview by Our Hong Kong Foundation
05/11/2021
Curious about our HKU Phase 1 Clinical Trials Centre? In the episode 1 of the interview, you will have a view of the Centre, and Mr. Henry Yau, our Managing Director, will also introduce HKU’s efforts and achievements in clinical research and novel drugs development.
https://www.youtube.com/watch?v=IWzwTAenZzA

In episode 2, you will know more about the strengths of Hong Kong in conducting clinical trials, as well as the challenges we need to overcome.
https://www.youtube.com/watch?v=LsT8brGP_F4

Phase 1 Study Proves Long-acting Formulation of Bupivacaine a Safe Means to Relieve Post-surgical Pain
29/09/2021
Phase 1 Study Proves Long-acting Formulation of Bupivacaine a Safe Means to Relieve Post-surgical Pain
29/09/2021
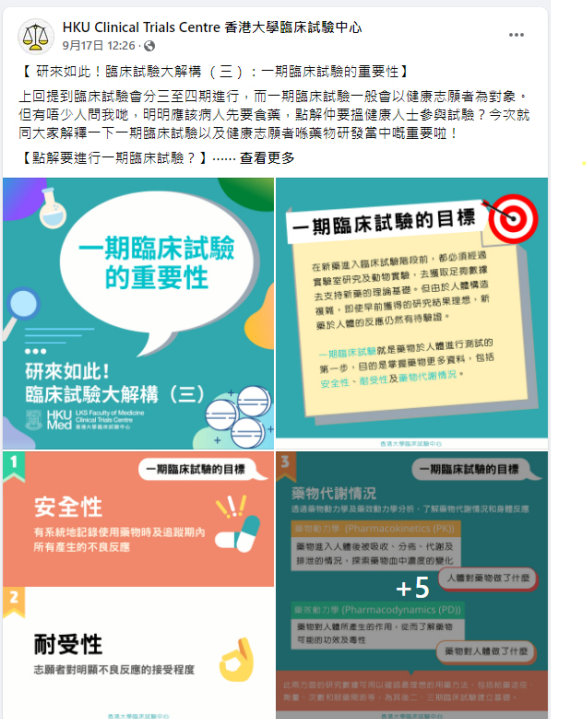
[HKU-CTC Public Education Series] Chapter 3: The Importance of Phase 1 Clinical Trials
17/09/2021
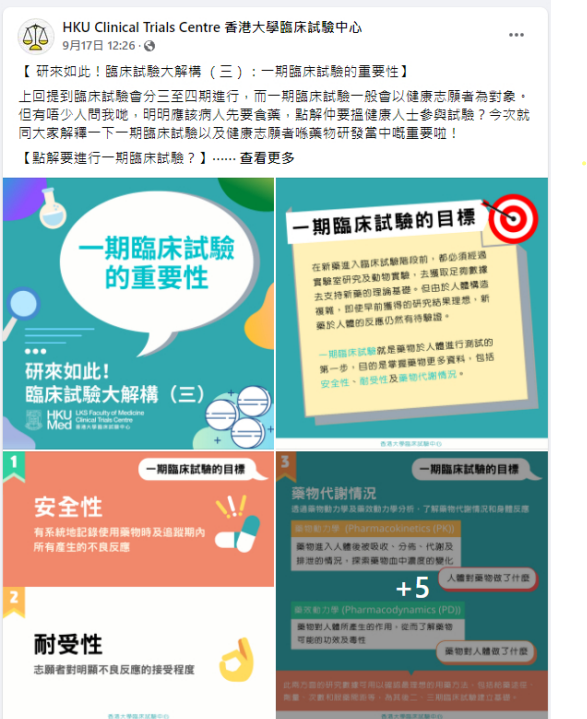
[HKU-CTC Public Education Series] Chapter 3: The Importance of Phase 1 Clinical Trials
17/09/2021
[Study News] Now News Report: Phase 1 clinical trial of HKU intranasal COVID-19 vaccine has commenced (May 15, 2021)
10/06/2021
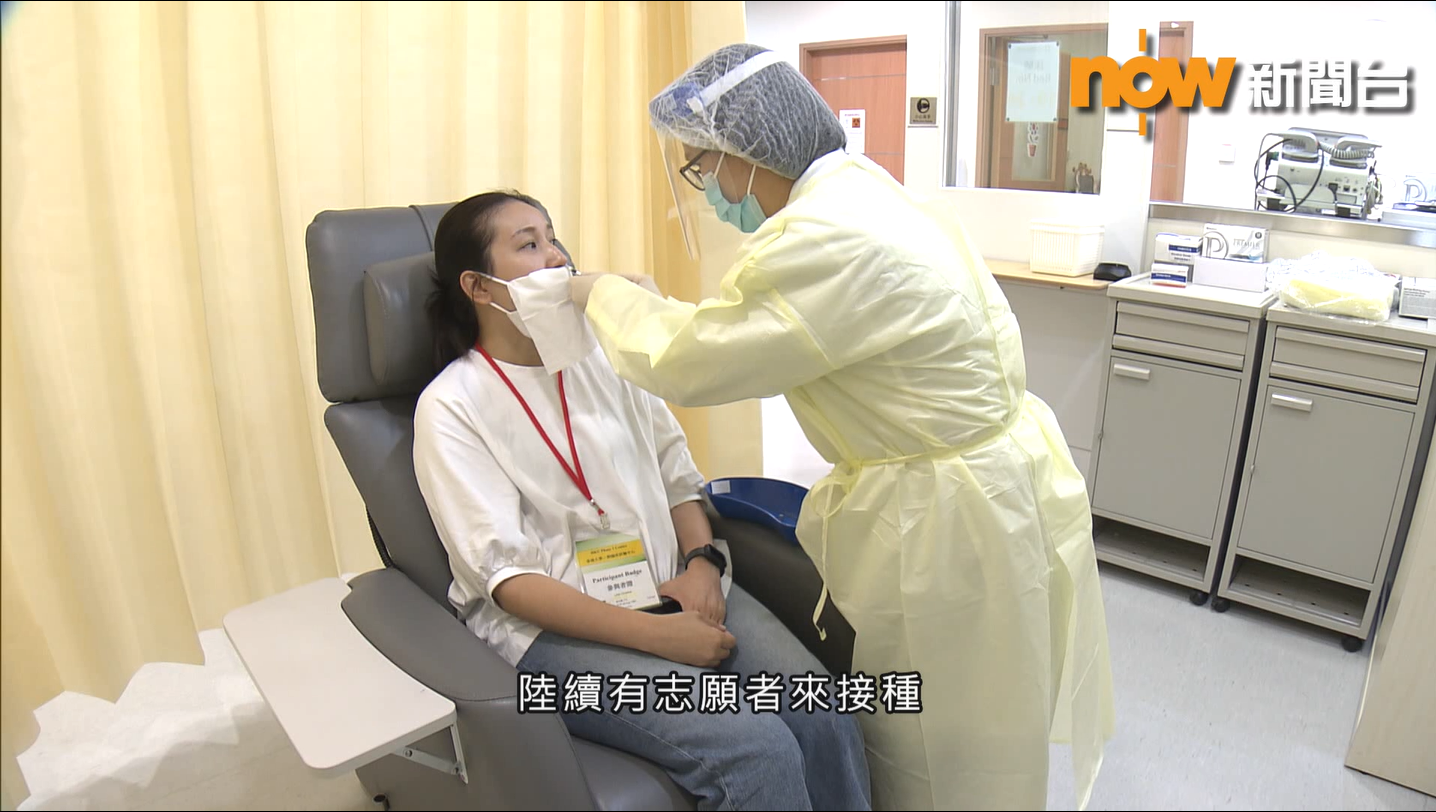
[Study News] Now News Report: Phase 1 clinical trial of HKU intranasal COVID-19 vaccine has commenced (May 15, 2021)
10/06/2021
Now News visited HKU Phase 1 Clinical Trials Centre earlier to produce a report on the process and progress of the phase 1 clinical trial of “VectorFlu ONE”. Here is the report by Now News (Chinese only):
Natural phytosterols prove to lower the low-density lipoprotein level and may reduce the risk of heart disease
24/05/2021
Natural phytosterols prove to lower the low-density lipoprotein level and may reduce the risk of heart disease
24/05/2021
High cholesterol level is prevalent in Hong Kong. About half of the population have hypercholesterolemia. It is one of the major risk factor for cardiovascular diseases. Professor Bernard Cheung of HKU Department of Medicine and Dr. Ching-Lung Cheung of HKU Department of Pharmacy and Pharmacology conducted a 3-week clinical trial in 2015.
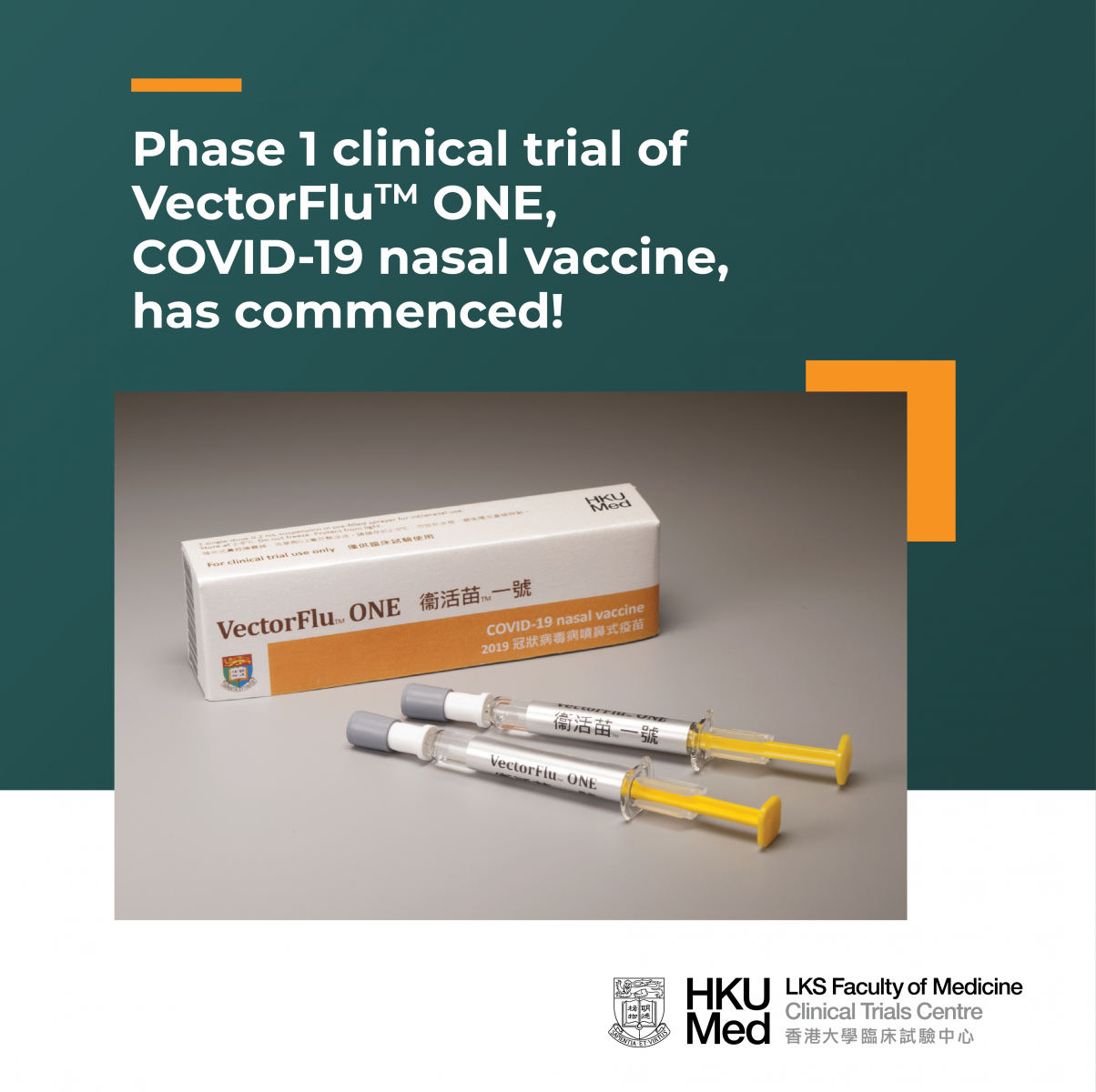
[Study News] Phase 1 clinical trial of VectorFlu ONE, COVID-19 nasal vaccine, has commenced!
14/05/2021

[Study News] Phase 1 clinical trial of VectorFlu ONE, COVID-19 nasal vaccine, has commenced!
14/05/2021
The phase 1 clinical trial of VectorFluTM ONE, the world’s first COVID-19 nasal vaccine developed by the research team of HKU Department of Microbiology, has commenced in March 2021 at the HKU Phase 1 Clinical Trials Centre. About 115 healthy volunteers will be recruited to evaluate the safety and immune responses of the vaccine.
VectorFluTM ONE is the first novel vaccine that is developed by Hong Kong and goes into human trial. The phase 1 trial will last for around 1.5 years. Part 1 of the trial has commenced in March 2021, where about 30 volunteers have already received one or two vaccinations. Part 2 will begin in July 2021 and 85 volunteers will be recruited. We are still looking for more volunteers to join.
Successful Phase 1 Study on Novel FcRn Inhibitor Offers New Direction for Treatment of Autoimmune Disease
04/05/2021
Successful Phase 1 Study on Novel FcRn Inhibitor Offers New Direction for Treatment of Autoimmune Disease
04/05/2021
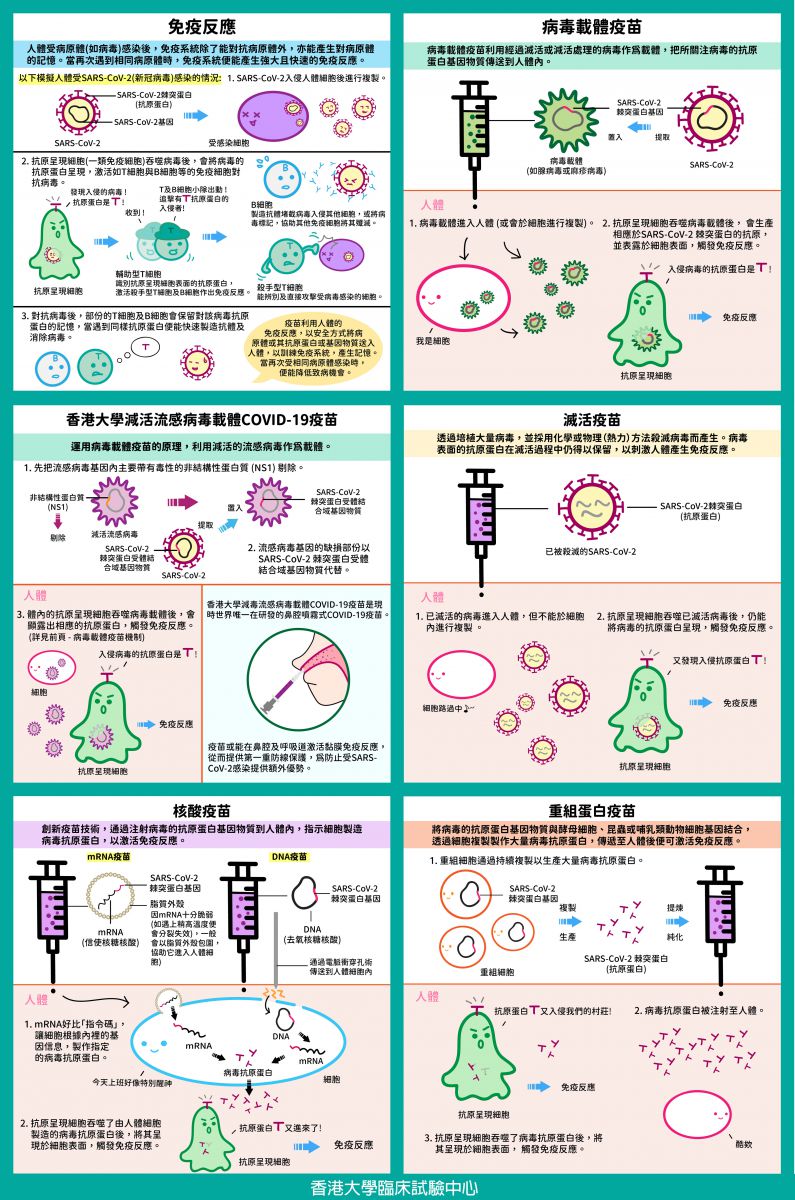
[Pharma Tips] Vaccine Technology for COVID-19 at a Glance
26/02/2021
Formulation of effective vaccine strategies for COVID-19 is not straight forward as the immune responses to COVID-19 in humans are still unclear. Application of various technologies would be necessary for developing safe and effective vaccines, as different vaccine technologies may be needed for different population. Immediately after the release of the genome sequence of SARS-CoV-2 in January 2020, multiple vaccine technologies have been investigated and proceeded to different stages of pre-clinical and clinical trials.
Below are the current major vaccine technologies for COVID-19 and their respective mechanisms:
Immune Response
When the human body is infected by a pathogen (e.g. virus), the immune system may fight the pathogen and develop memory towards it. When the same pathogen is encountered again, the immune system may establish strong and rapid immune responses.
By utilizing the human immune responses, vaccines may train the immune system to recognize a pathogen by safely delivering the pathogen or its antigen or genetic material into the human body. Therefore, when the human body is exposed to the same pathogen again, the chance or severity of infection may be lowered.
5 Types of Vaccine Technology
Viral Vector Vaccine
Viral vector vaccine uses a virus without virulence as a vehicle to deliver the genetic material of the antigens of the virus of interest into human body. The genetic material of the viral antigens uses human cells’ machinery to produce the respective antigens and presented on the cell surface, the immune system will then react.
Viral vector vaccine has been extensively studied to fight against infectious diseases, including influenza, HIV and Ebola etc. The most common viral vector platform used for vaccine production is adenoviral vector. A recently approved COVID-19 viral vector vaccine by regulatory authorities uses adenovirus as vehicle to deliver the genetic material of the spike protein of SARS-CoV-2 into human cells, at where the spike protein is expressed, and trigger the immune system to produce antibodies and T-cell responses.
HKU’s Flu-based Live Attenuated Viral Vector COVID-19 Vaccine
The HKU’s flu-based live attenuated viral vector vaccine uses attenuated influenza virus as the viral vector. Its key virulent element, non-structural protein NS1 is removed which makes the influenza vector severely attenuated, and this knock-out region is replaced by the genetic material of the receptor binding domain (RBD) of SARS-CoV-2 spike protein. The RBD of SARS-CoV-2 spike protein will eventually trigger the immune response following the above viral vector vaccine mechanism.
HKU’s flu-based live attenuated viral vector COVID-19 vaccine is the only intranasal COVID-19 vaccine under development worldwide. It might have additional benefit of inducing mucosal immune response in the nasal cavity and respiratory tract, and offer a first line protection against SARS-CoV-2 infection. The phase 1 clinical trial of this vaccine will be conducted in HKU Phase 1 Clinical Trials Centre, and is under preparation by Department of Microbiology, Department of Medicine and Clinical Trials Centre of the University of Hong Kong.
Inactivated Virus Vaccine
Through cultivation of a large amount of viruses and killing the viruses by chemical or physical (heat) technology, inactivated virus vaccine is produced. Immune responses are triggered by the preserved antigens presented on the viral surface. Since inactivated viruses could not replicate by themselves, the quantity of virus required will be large, which may result in higher dosage level and multiple vaccinations. This vaccine production technique has been long established and been applied to vaccines against influenza, polio and rabies etc. Currently, some COVID-19 vaccines approved by regulatory authorities have adopted this inactivated virus vaccine technique.
Nucleic Acid Vaccine
Nucleic acid vaccine against infectious diseases is an innovative vaccine technology. Currently, mRNA (messenger RNA) and DNA vaccine technologies are under active development worldwide against COVID-19. Both technologies aim at triggering immune responses through injecting genetic materials coded for the spike protein of SARS-CoV-2 into human bodies.
mRNA vaccine: Encased in a lipid coat, mRNA carrying the genetic information of viral antigens is injected into human body, where it instructs ribosomes to synthesize viral antigens. Immune responses are then triggered.
DNA vaccine: The DNA plasmid with the genetic information of the viral antigens is transmitted into human cells through electroporation, and then is translated into viral antigens through mRNA. Immune responses are then triggered.
Recombinant Protein Vaccine
The genetic materials of the viral antigens are delivered into yeast cells, insect (baculovirus) or mammalian cells to express viral antigens. These recombinant cells continue to multiply and produce large quantity of viral antigens. The viral antigens will be extracted from the cells and purified, of which recombinant protein vaccine is produced.
Immune responses will be triggered by the viral antigens once the vaccine is injected into human body. This technology has been adopted for producing Hepatitis B vaccine and Human Papillomavirus vaccine. Through the expression of antigens of SARS-CoV-2 (e.g. spike protein), recombinant protein vaccine could be developed for fighting against COVID-19.