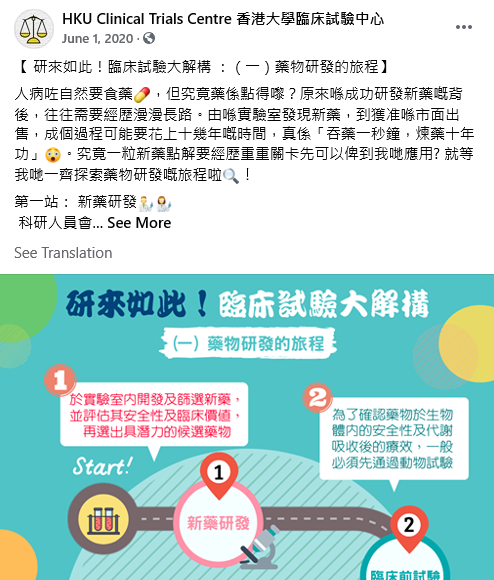
[Study Completion] Successful Completion of Generic Drug Study Indicated for Hyperlipidemia!
30/12/2020

[Study Completion] Successful Completion of Generic Drug Study Indicated for Hyperlipidemia!
30/12/2020
The recruitment of this study started in July 2020 and 52 healthy volunteers were recruited. This clinical study consisted of 2 parts (fasted and fed), and each volunteer only joined either one part. The duration of each part was around 4-6 weeks, including screening, confinement and follow up visits. Although the study process was slightly postponed due to the COVID-19 pandemic, thanks to the genuine support from our volunteers and the effort of our HKU professional research team, the project was completed safely and efficiently in November 2020.

[Study Completion] Phase 1 Clinical Trial of a New Autoimmune Disease Drug Was Completed!
01/12/2020

[Study Completion] Phase 1 Clinical Trial of a New Autoimmune Disease Drug Was Completed!
01/12/2020
HKU Clinical Trials Centre was delegated to research on a novel drug for treating autoimmune diseases. Phase 1 trial of the drug was conducted in Canada earlier but the research purpose was to evaluate the metabolism and safety of the drug at dose escalation in Chinese population.
Recruitment started in May 2019 and 24 qualified healthy volunteers were selected after screening. The 18-week research was divided into 3 cohorts and each volunteer only joined one of them. The volunteers received injection during their 6-day-5-night stay in the Centre. As the duration of overnight stay was long, two workshops were organized, namely Spark Chamber and Mini Neon Light DIY Workshops.
The clinical study was successfully completed in November 2020.

[Centre Activity] The 6th International Clinical Trial Center Network (ICN) Steering Board Meeting and Annual General Meeting 2020
27/11/2020
[Centre Activity] The 6th International Clinical Trial Center Network (ICN) Steering Board Meeting and Annual General Meeting 2020
27/11/2020
HKU-CTC successfully hosted the 6th ICN Steering Board Meeting and Annual General Meeting 2020 on 24-25 November, 2020. Participants engaged actively and had vivid discussions – even though the meetings were moved online due to the pandemic.
At the Steering Board Meeting, Mr. Henry Yau, Managing Director of HKU-CTC, was elected the new ICN Chairperson. He will work with the new Vice Chairperson, Dr. Christiane Blankenstein of Munich Study Center, Technical University of Munich, the ICN Steering Board, and the ICN Operations Team to contribute to the further development of ICN in the coming two years. The Steering Board also formulated a strategic plan towards realization of the values of “Global, Excellence, Harmonization, Impacts”.
The next ICN Steering Board Meeting and Annual General Meeting will be hosted by Cambridge Clinical Trials Unit (CCTU), Cambridge University Hospitals NHS Foundation Trust in November 2021.

Combined Use of Immunotherapy Drugs Improves Clinical Outcomes for Patients with Advanced Hepatocellular Carcinoma and Received FDA Approval as a Second-line Therapy for HCC
10/11/2020
Combined Use of Immunotherapy Drugs Improves Clinical Outcomes for Patients with Advanced Hepatocellular Carcinoma and Received FDA Approval as a Second-line Therapy for HCC
10/11/2020
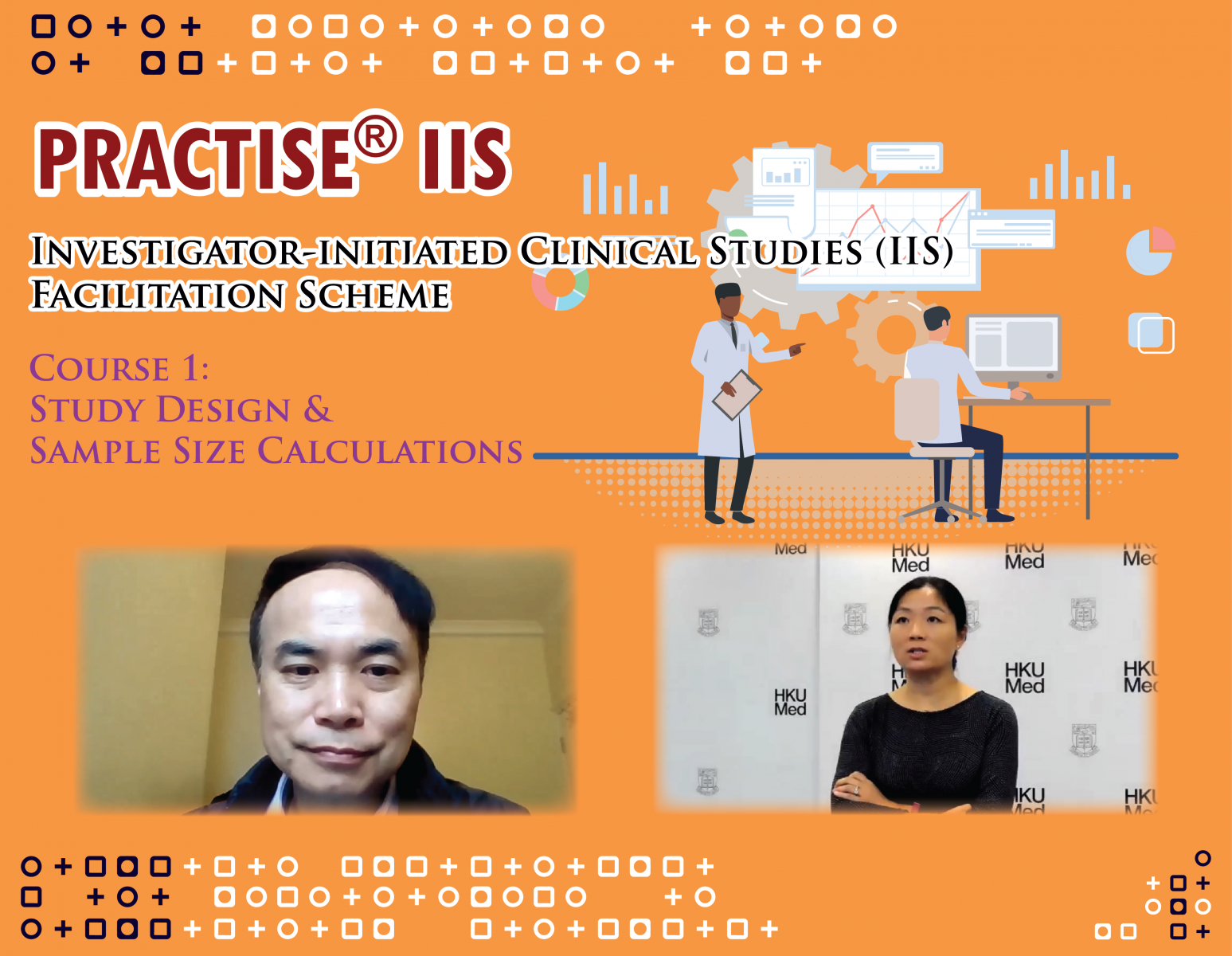
[Centre Activity] PRACTISE® IIS - Training Course for Investigator-Initiated Clinical Studies
31/08/2020
[Centre Activity] PRACTISE® IIS - Training Course for Investigator-Initiated Clinical Studies
31/08/2020
Clinical research is an essence of medical advancement and a cornerstone of a robust health care system. In general, there are two types of clinical research – industry-sponsor studies and investigator-initiated studies (IISs) – whereas those initiated by investigators are increasingly important nowadays for its variety and diversity. They also bridge the research gaps on areas which are rarely covered by the industry. Facilitation of IISs is therefore a focus of The University of Hong Kong Clinical Trials Centre (HKU-CTC). In order to encourage investigators to initiate more high impact IISs, HKU-CTC has recently launched PRACTISE® IIS – a training programme addressing the specific considerations for IISs.
The first course was completed by way of an online seminar on 28th August, 2020. Professor Ian Wong, Head of Department of Pharmacology and Pharmacy, and Dr. Helen Zhi, Director of Biostatistics and Clinical Research Methodology Unit, shared their practical tips and experience in designing and managing big data research and clinical studies with over 300 participants and received enthusiastic response. CTC will continue to organize other courses on IISs.
Immunotherapy Drug Proven Safe and Effective New Treatment for Asian HCC Patients
03/06/2020
Immunotherapy Drug Proven Safe and Effective New Treatment for Asian HCC Patients
03/06/2020
[Study News] Clinical Studies of COVID-19
05/03/2020
To help identify possible treatments for tackling COVID-19, The University of Hong Kong Clinical Trials Centre join forces with Queen Mary Hospital, Prince of Wales Hospital, Princess Margaret Hospital and the manufacturer of remdesivir, an investigational antiviral drug, in arranging for two clinical trials targeting for the treatment of moderate to severe COVID-19 patients. With a common goal of accelerating the preparation for the trials, the parties have been working hard in the past few weeks and quickly obtained the approvals from the research ethics committees. It is anticipated that the trials will be initiated within this month (i.e. March 2020).

[Centre Activity] Celebrating Chinese New Year with Clinical Trial Supporters
20/02/2020
HKU-CTC set up a Chinese New Year Booth at Queen Mary Hospital on 21st January, 2020, to celebrate Chinese New Year with the supporters. Dr. Hong, one of the CTC Panda Ambassadors, visited the booth on that day. He gave out calendar cards and fai-chuns to the visitors. Visitors who participated in the quiz game at the booth and answered all questions correctly were given specially designed red packets as well. The responses were enthusiastic. Here are some highlights of the event: